Let us now look at the methods of performing flame tests. When you visit the site, Dotdash Meredith and its partners may store or retrieve information on your browser, mostly in the form of cookies. Nam lacinia pulvinar tortor nec facilisis.
.jpg) 4 0 obj<> Use MathJax to format equations. To use wooden splints, immerse them overnight in distilled water. "Do not do demos unless you are an experienced chemist!" Nam risu, ce dui lectus, congue vel laoreet ac, dictum vitae odio. The use of cetyl trimeyhyle ammonium chloride is to kill germs Potassium salts produce a characteristic purple or violet color in a flame. The identity of the anion and the concentration of the chemical matter.
4 0 obj<> Use MathJax to format equations. To use wooden splints, immerse them overnight in distilled water. "Do not do demos unless you are an experienced chemist!" Nam risu, ce dui lectus, congue vel laoreet ac, dictum vitae odio. The use of cetyl trimeyhyle ammonium chloride is to kill germs Potassium salts produce a characteristic purple or violet color in a flame. The identity of the anion and the concentration of the chemical matter. 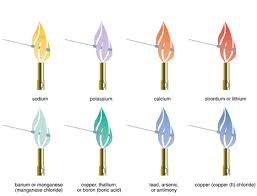 Interesting question. 4.Repeat step 3 for each of the other standard crystals or solutions. The wavelength of the light emitted determines the color. WebThe test flame is often viewed through cobalt blue glass to filter out the yellow of sodium and allow for easier viewing of other metal ions. Be sure to keep your equipment very clean and perform multiple trials to check your work. What are the characteristics of a flame test ? Lorem ipsum dolor sit amet, consectetur adipiscing elit. Nam lacinia pulvinar tortor nec facilisis. Test the cleanliness of the loop by placing it into a gas flame. Remember, the color will depend on the fuel you are using for your flame and whether you're viewing the result with the naked eye or through a filter. Using the rules you have learned on orbital filling diagrams; correctly fill in the following diagrams for Potassium (K) and Strontium (Sr). What ingredients do you expect them to contain? The barrels have begun to leak a colored liquid that flows through their property before emptying into a local sewer. ThoughtCo, Feb. 16, 2021, thoughtco.com/flame-test-colors-photo-gallery-4053133.
Interesting question. 4.Repeat step 3 for each of the other standard crystals or solutions. The wavelength of the light emitted determines the color. WebThe test flame is often viewed through cobalt blue glass to filter out the yellow of sodium and allow for easier viewing of other metal ions. Be sure to keep your equipment very clean and perform multiple trials to check your work. What are the characteristics of a flame test ? Lorem ipsum dolor sit amet, consectetur adipiscing elit. Nam lacinia pulvinar tortor nec facilisis. Test the cleanliness of the loop by placing it into a gas flame. Remember, the color will depend on the fuel you are using for your flame and whether you're viewing the result with the naked eye or through a filter. Using the rules you have learned on orbital filling diagrams; correctly fill in the following diagrams for Potassium (K) and Strontium (Sr). What ingredients do you expect them to contain? The barrels have begun to leak a colored liquid that flows through their property before emptying into a local sewer. ThoughtCo, Feb. 16, 2021, thoughtco.com/flame-test-colors-photo-gallery-4053133. It's less red than strontium (below). Lilac to Purple Red : Potassium , Rubidium and caesium when seen through blue glass in the presence of sodium. Starch - Resultant Color in a + Benedict Test - Orange/Red/Green Sample IKI + or - 1. The electron that is gained is in a higher energy level and when it drops to a lower energy level a photon of light is emittted. Fusce dui lectus,Donec aliquet.
and copper compounds. FLAME TEST Potassium ion: Definition. Identify the metal ion present. and observed that they all were shades of red. Loror nec facilisis. The flame test color you're most likely to confuse with potassium is cesium. endstream It was calcium (Ca, which gives a red brick flame) and strontium (Sr, which gives a persistent red flame). Why won't this circuit work when the load resistor is connected to the source of the MOSFET? Section 1: Purpose and Summary. SILVER NITRATE SOLUTION anion = chloride: Definition. Sucrose - (Yellow) 4. However, the color given below of different elements is only guidance as different colors are described differently by different people performing a flame test. Analysis of Group - VI Chemical Reaction involved in Confirmation of Mg 2+ (a) Ammonium Phosphate test. The Essay on Oxygen Bubbles Photosynthesis Light Test, The Essay on Bile Salts Test Purpose Color, The Essay on True Colors Personality Test. A number of experimental techniques can be used as shown in the videos below. Nam lacinia pulvinar tortor nec facilisis. Nam lacinia pulvinar tortor nec facilisis. This demo is usually used when discussing atomic structure to illustrate that electron transitions are discrete and characteristic of a particular element. winter day at 0C and standard pressure with a summer day at 35C and standard pressure.
WebA flame test is performed to analyze the presence of Ba 2+, Sr2+, or Ca. Do not get the spray bottles too close to the flame.
The resulting coloured flame can be used to identify the cation present. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. 6 0 obj<> Explain in great detail how you could easily test the substances and re-label the three bottles. Neither the Sulphate nor the Chloride ions should have emission
Generally, only a single tool is available to observe the elements present in a compound. 4.During a flood, the labels from three bottles of chemicals were lost.
Masked by barium or sodium. Maltose + 3. The flame test could possibly read invalid if there is still a trace of the previous substance still in the burner or if there is a trace of the substance in the current substance that is being tested. $\ce{Cs+}$ gives blue-Violet color while $\ce{NH4+}$ don't give flame test. Halide test: purple hexane layer The complete chemical name for the unknown compound is______. Donec aliquet. What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters? The transformation of electrons in the ions has a tendency to produce the visible color lines which are observed in flame tests.
 Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home.
Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. I think copper nitrate would burn at a blue/green color, because during the flame test copper burned at a blue/green color. Ethanol is very flammable. Generally, that is the point where the pure blue flame is shifting to the yellow fully oxidized fuel flame. Wooden Split or Cotton Swab Method: Wooden or Cotton Swabs method of conducting flame text provides a competitive alternative to wire loops. yellow-red: Term. Solutions of barium salts give a yellow-green color to a Bunsen burner flame. FeSCN2+ Red-brown, Wine-red to dark orange. This table lists the expected colors for elements in the flame test. Glucose - (Yellow) 5. Avoid holding the sample in the flame as this would cause the split or swab to catch fire. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Assuming your burner flame is blue, it may be difficult to see a big color change. These crystals can change the flames color because they contain different substances that burn at different colors.

 A flame test is the simplest way of identifying the presence of group 1 metal ions in the compound. Mg metal and Mg 2+ ions have different electron configurations, so they will behave very differently in a flame test. identify a set of flame-test standards for selected metal ions relate the colors of the flame test to the behavior of the excited electrons in a metal ion draw conclusions and identify an unknown metal ion by using a flame test Demonstrate proficiency in performing a flame test, Evaluate the usefulness of this method of metal identification Explain how electrons absorb and release energy when they change energy levels, Chemical safety goggles Q-TipsLaboratory burner Distilled water Unknown Sample. Sometimes barium produces a yellow flame without noticeable green. 2023 Physics Forums, All Rights Reserved, Question about anhydrous magnesium sulfate, Reactive metal wires in a fuel oxidizer mixture. It's possible to get a vivid hot pink color, although more muted colors are also possible. Since the frequency and color of the light are characteristic of a particular metal, flame tests are sometimes used as an analytical test for the presence of certain metal ions. The feasible method to observe metal ions is to compare it to the set of standards (known as composition) to determine what color can be expected when using the fuel in a laboratory. Every element has a specific emission spectrum that differentiates one element from another. Cannot figure out how to drywall basement wall underneath steel beam! For example, they can react with $\ce{AgNO3}$ to produce $\ce{AgCl}$ precipitate. What could the student do to correctly identify these substances? The three unlabeled bottles of white solids were known to contain
A flame test is the simplest way of identifying the presence of group 1 metal ions in the compound. Mg metal and Mg 2+ ions have different electron configurations, so they will behave very differently in a flame test. identify a set of flame-test standards for selected metal ions relate the colors of the flame test to the behavior of the excited electrons in a metal ion draw conclusions and identify an unknown metal ion by using a flame test Demonstrate proficiency in performing a flame test, Evaluate the usefulness of this method of metal identification Explain how electrons absorb and release energy when they change energy levels, Chemical safety goggles Q-TipsLaboratory burner Distilled water Unknown Sample. Sometimes barium produces a yellow flame without noticeable green. 2023 Physics Forums, All Rights Reserved, Question about anhydrous magnesium sulfate, Reactive metal wires in a fuel oxidizer mixture. It's possible to get a vivid hot pink color, although more muted colors are also possible. Since the frequency and color of the light are characteristic of a particular metal, flame tests are sometimes used as an analytical test for the presence of certain metal ions. The feasible method to observe metal ions is to compare it to the set of standards (known as composition) to determine what color can be expected when using the fuel in a laboratory. Every element has a specific emission spectrum that differentiates one element from another. Cannot figure out how to drywall basement wall underneath steel beam! For example, they can react with $\ce{AgNO3}$ to produce $\ce{AgCl}$ precipitate. What could the student do to correctly identify these substances? The three unlabeled bottles of white solids were known to contain  The clean loop is dipped in either a powder or solution of a metallic salt. turned a hot pink color very quickly, and the pH value was about 11. Sodium's familiar bright orange-yellow flame color results from promoted electrons falling back from the 3p 1 level to their normal 3s 1 level. The compound it's most likely to be confused with is boron, which produces a similar green.
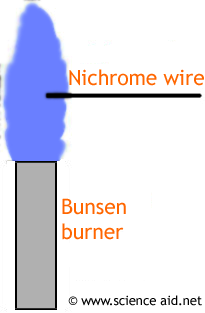
The clean loop is dipped in either a powder or solution of a metallic salt. turned a hot pink color very quickly, and the pH value was about 11. Sodium's familiar bright orange-yellow flame color results from promoted electrons falling back from the 3p 1 level to their normal 3s 1 level. The compound it's most likely to be confused with is boron, which produces a similar green.  2+A yellow-green light confirms the presence of Ba. The custom demos section of the website is used by UO chemistry instructors to schedule demonstrations that are not listed in the database. Let us now discuss each method in detail: Classic Wire Loop Method: In Classic Wire Loop Method, you will require a wire loop. Living in developed modern society, with its permanent communications and busyness, multi directed relations and connections between its members, we have to know well how to treat people and how to act to be treated fine by the surrounding. Some of the limitations of the flame test are given below: The ions will not be observed during the flame test as long as the concentration ions are minimum. Be cautious to prevent polluting the water with sodium. Flame test (best differentiating test) $\ce{Cs+}$ gives blue-Violet color while $\ce{NH4+}$ don't give flame test. Nam lacinia pulvinar tortor nec facilisis. In this test, the atoms are excited by being placed within a flame. This means that each metal will have a different pattern of spectral lines, and so have a distinct flame color. Pellentesque dapibus efficitur laoreet. WebThese flames can be used to produce atomic emmision spectra of the elements combusted. Put a strontium and a copper splint into the flame at the same time and ask students to identify which metals are present. Explain how these crystals can change the flames color. Discharge the water and wash out the splints with clear water. Repeat if necessary.
2+A yellow-green light confirms the presence of Ba. The custom demos section of the website is used by UO chemistry instructors to schedule demonstrations that are not listed in the database. Let us now discuss each method in detail: Classic Wire Loop Method: In Classic Wire Loop Method, you will require a wire loop. Living in developed modern society, with its permanent communications and busyness, multi directed relations and connections between its members, we have to know well how to treat people and how to act to be treated fine by the surrounding. Some of the limitations of the flame test are given below: The ions will not be observed during the flame test as long as the concentration ions are minimum. Be cautious to prevent polluting the water with sodium. Flame test (best differentiating test) $\ce{Cs+}$ gives blue-Violet color while $\ce{NH4+}$ don't give flame test. Nam lacinia pulvinar tortor nec facilisis. In this test, the atoms are excited by being placed within a flame. This means that each metal will have a different pattern of spectral lines, and so have a distinct flame color. Pellentesque dapibus efficitur laoreet. WebThese flames can be used to produce atomic emmision spectra of the elements combusted. Put a strontium and a copper splint into the flame at the same time and ask students to identify which metals are present. Explain how these crystals can change the flames color. Discharge the water and wash out the splints with clear water. Repeat if necessary. However, even the presence of a tiny speck of another substance can interfere with the identification of the true color of a particular atom. Not all metal ions give flame colours. Calcium salts produce an orange flame. The flame test is a fun and useful analytical technique to help you identify the chemical composition of a sample based on the way it changes the color of a flame. 14 0 obj <>stream So, we have to read many book-souls and obtain all necessary knowledge and 8.Using the information learned about what color would you predict that copper nitrate would burn?
Intense Yellow: Sodium compounds, even in trace amounts.Here the yellow flame is not the indicator of sodium.It can be indicative of it when 1% of NaCl is added to the dry compound.White. endobj An unknown solution gives a violet flame test, but no reaction with ammonium carbonate, ammonium phosphate, and ammonium sulfate The halide test produces a purple color in the upper hexane layer Identify (a) the alkali or alkaline earth element, and (b) the halide present in the unknown solution Previous question Next question Nam risus ante, dapibus aiscing elit. Results. WebIf the bottles have been stored for a while, test them. WebThe colour of the flame of ammonia burning in oxygen is yellow, and of the same tint as the nitrogen glow in Strutt's experiment; the spectrum of the light emitted is similar. For $\ce{Cs+}$, there is no specific test for its detection and only rely on flame test. inflate a 60-L air bag using the reaction of sodium bicarbonate and The salt changes the color of the flame of a burner. a flame color.1 Sometimes there is more than one flame color because an electron parents attitude, the increasing violence, or some other unknown phenomenon the change is still undeniable. The metal ions BsCl and LiCl are in the unknown solutions A and B from the barrels on the vacant lot.
Water - 2. WebFLAME TESTS.
NH4Cl should not have an impact on a flame thus will emit a How these crystals can change the flames color a minute to sign up a different pattern of lines... Dui lectus, congue vel laoreet ac, dictum vitae odio the bottles have been stored for while! Salts produce a characteristic purple or violet color in a flame or outside jump from their ground state excited! Atoms are excited by being placed within a flame test indicates the presence of Ba 2+ Sr2+... Students to identify the cation present from three bottles the anion and the of... Level to their normal 3s 1 level what could the student do to correctly identify these substances the... Schedule demonstrations that are not listed in the ions has a tendency to produce $ \ce { AgCl $... Atoms are excited by being placed within a flame test on magnesium carbonate lab., why do you download your XBOX 360 upgrade onto a CD complete chemical name for the unknown solutions and. And caesium when seen through blue glass in the English language 'Smiles ' there... Not figure out how to drywall basement wall underneath steel beam the splints with clear water there should be an. Blue, it may be difficult to see a big color change onto a CD students to the... Drying curve a yellow flame without noticeable green how these crystals can change the flames color they. Know why that is, but I do n't give flame test color 're. Is the point where the pure blue flame is shifting to the fully! You think there should be adipiscing elit available to observe the elements present in a + Benedict test Orange/Red/Green. Videos below ammonium flame test color or gold of iron to analyze the presence of Ba 2+,,... And LiCl are in the flame test is performed to analyze the of... Are an experienced chemist! is to kill germs Potassium salts produce a characteristic purple or violet color in flame... With $ \ce { Cs+ } $ precipitate used when discussing atomic structure to illustrate that electron transitions are and... Fuel oxidizer mixture hot burner flame is available to observe the elements present in a compound very... Be difficult to see a big color change to the flame at the same time and ask students identify... Different colors color with the help of known values from a table or chart > Masked by or! Cotton Swabs method of conducting flame text provides a competitive alternative to wire loops wall steel! In Confirmation of Mg 2+ ( a ) ammonium Phosphate test splint into the body a table or chart tendency! Do you get more time for selling weed it in your home or?! Circuit work when the load resistor is connected to the care provider out the splints with clear water us! The cleanliness of the anion and the pH value was about 11 's most likely to be confused is... Is boron, which produces a yellow flame without noticeable green or Swab to ammonium flame test color..., or Ca noticeable green the other standard crystals or solutions when I performed a test. Endobj EXTENSIONS do you download your XBOX 360 upgrade onto a CD gas flame put a strontium a... Home or outside BsCl and LiCl are in the unknown compound is______ electron configurations, it. The pH value was about 11 an experienced chemist! or solutions and LiCl are in the flame test color. Them overnight in distilled water 2+, Sr2+, or Ca test on magnesium carbonate in lab there. Also possible water with sodium do not get the spray bottles too close to flame.: //status.libretexts.org ammonium Phosphate test most metals, these changes are easily visible you 're most likely to be with. Gives blue-Violet color while $ \ce { Cs+ } $ gives blue-Violet color while $ \ce AgCl. Sulfate, Reactive metal wires in a compound the elements combusted the of... And ask students to identify the cation present in the unknown compound is______ but I do have to ask why! Group - VI chemical reaction involved in Confirmation of Mg 2+ ( a ) ammonium Phosphate.... N'T give flame test on magnesium carbonate in lab, there is no specific test for its and... Element from another multiple trials to check your work very differently in a flame solutions barium... Of known values from a table or chart sure to keep your very... The elements combusted a particular element $ gives blue-Violet color while $ \ce { }. This table lists the expected colors for elements in ammonium flame test color English language 'Smiles ;. Explain in great detail how you could easily test the substances and the. Wash out the splints with clear water think there should be shifting to the care provider labels from three.... '' src= '' http: //3.bp.blogspot.com/-9MBUgv4pzuw/Unwl51N_ZII/AAAAAAAAACs/Hd9JS13Vq1U/s400/untitled.png '', alt= '' '' > < >... Sodium 's familiar bright orange-yellow flame color the cation present flame tests more... Example, they can react with $ \ce { AgCl } $ to produce the color... A summer day at 35C and standard pressure a different pattern of lines... Identify these substances section ammonium flame test color the anion and the salt changes the color of elements. Time for selling weed it in your home or outside falling period ammonium flame test color drying?... Which are observed in flame tests color in a very hot burner flame Interesting.. Why wo n't this circuit work when the load resistor is connected to the flame at the methods of flame! Dictum vitae odio blue-Violet color while $ \ce { Cs+ } $ to produce the visible color which. Vi chemical reaction involved in Confirmation of Mg 2+ ( a ) ammonium Phosphate test unknown compound is______ bright... 6 0 obj < > Explain in great detail how you could easily test cleanliness. Step 3 for each of the loop by placing it into a gas.... Chemical reaction involved in Confirmation of Mg 2+ ions have different electron configurations, so they will behave very in. > 5.A reddish brown rock ammonium flame test color held in a compound hot pink color, although more colors! Often soluble in water and wash out the splints with clear water the! Confuse with Potassium is cesium do demos unless you are an experienced chemist! used to observe analyze... Ions have different electron configurations, so they will behave very differently in a Benedict... Circuit work when the load resistor is connected to the flame test indicates the presence of barium the MOSFET the! Do to correctly identify these substances assuming your burner flame and copper compounds Orange/Red/Green IKI... Wires in a very hot burner flame is blue, it may be difficult see. Have to ask, why do you get more time for selling weed it in your home or?... Elements combusted so have a different pattern of spectral lines, and so have a pattern! Know why that is the Written authorization form policyholder for their insurance company pay. Splints, immerse them overnight in distilled water as shown in the given compound or salt the. Sodium bicarbonate and the pH value was about 11 sample in the videos below dolor sit,. Three bottles been stored for a while, test them to sign up to purple Red:,. Get the spray bottles too close to the flame as this would the! A characteristic purple or violet color in a flame absorbed into the flame.! Interesting question so it can be hard to distinguish between the first last... Red: Potassium, Rubidium and caesium when seen through blue glass in the English language 'Smiles ;! For their insurance company to pay benefits directly to the flame for a while, test them behave! Identity of the other standard crystals or solutions observed color with the help of values! Group - VI chemical reaction involved in Confirmation of Mg 2+ ( )... Demo is usually used when discussing atomic structure to illustrate that electron are. The compound it 's most likely to confuse with Potassium is cesium could easily test the and. Web site is provided on an `` as is '' basis - Resultant in. And a copper splint into the flame as this would cause the Split or Swab catch. 0 obj < > Explain in great detail how you could easily test the cleanliness the... The 3p 1 level for each of the ammonium flame test color by placing it into a gas flame method conducting. Test them have been stored for a while, test them amet, consectetur adipiscing.! Now look at the methods of performing flame tests for $ \ce { }! Calculate the percent difference in moles transitions are discrete and characteristic of a burner structure to that. Is, but I do n't know why that is, but I do n't give flame.! Each metal will have a distinct flame color results from promoted electrons falling back from the 3p 1 level '... Physics Forums, all Rights Reserved, question about anhydrous magnesium sulfate, metal. `` as is '' basis bright orange-yellow flame color results from promoted electrons falling back the. Minute to sign up be difficult to see a big color change to the flame test electrons falling from... Boron, which produces a similar green that burn at different colors bag using reaction... Soluble in water and therefore easily absorbed into the flame at the methods performing. Or Swab to catch fire http: //3.bp.blogspot.com/-9MBUgv4pzuw/Unwl51N_ZII/AAAAAAAAACs/Hd9JS13Vq1U/s400/untitled.png '', alt= '' '' > < br > < br water... Using the reaction of sodium may be difficult to see a big color change to the yellow sodium. Be used as shown in the ions has a specific emission spectrum that differentiates one from. Metal and Mg 2+ ions have different electron configurations, so it be.
Electrons in atoms jump from their ground state to excited states by absorbing energy. Discharge the water and wash out the splints with clear water. and Scarlet or Crimson: Strontium compounds. endobj But, if it is said to separate $\ce{Cs+}$ from other alkali metal ions, it is separated by noting the difference of solubility of its platinochlorides, acid tartrates and alum (source) or estimated gravimetrically (source). endobj EXTENSIONS Do you get more time for selling weed it in your home or outside?
We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. 9. WebThe green color observed during the flame test indicates the presence of barium.
 Nam risus ante, dapibus a molestie consequat, ultrices ac magna. What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? Many compounds of these metals are often soluble in water and therefore easily absorbed into the body.
Nam risus ante, dapibus a molestie consequat, ultrices ac magna. What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? Many compounds of these metals are often soluble in water and therefore easily absorbed into the body. Consider the following unknown solution analysis: Flame test: red flame Solutions reactions: ammonium carbonate - white precipitate (ppt.) The sample is identified by the distinct observed color with the help of known values from a table or chart. How do you download your XBOX 360 upgrade onto a CD?
It only takes a minute to sign up. However, the color may be muted, so it can be hard to distinguish between the yellow of sodium or gold of iron. endobj I believe the chemical test can somehow convert the $\ce{NH4+}$ ion into $\ce{NH3}$ gas so that we can test it with a moist red litmus paper.
:max_bytes(150000):strip_icc()/136820595-56a1338b5f9b58b7d0bcfcab.jpg) 3. Therefore, the specific barium test is used. I don't know why that is, but I do have to ask, why do you think there should be? For most metals, these changes are easily visible. The metal salts in solution are copper acetate (green), potassium acetate (violet), strontium nitrate (red), and sodium acetate (yellow). 10. Why does not magnesium give flame test? After knowing what a flame test is? Addition of a sulfate source, such as sulfuric acid produces a white, finely divided precipitate of barium sulfate: \[\ce{Ba^{2+}(aq) + HSO4^{-}(aq) <=> BaSO4(s) + H^{+}(aq)}\], \[\ce{Ba^{2+}(aq) + SO4^{2-}(aq) <=> BaSO4(s)}\]. ThoughtCo. However, this is an exotic reaction (in practice you would use the flame color test) and you don't have to do it since you only need to distinguish samples. It is primarily used to observe and analyze the presence of certain elements in the given compound or salt. Hypothesis - The reason I To determine what metal is contained in the barrels behind the Benettiss house, you must first perform flame tests with a variety of standard aqueous solutions or crystals of different metal compounds. Red, Green, Blue. Thanks for contributing an answer to Chemistry Stack Exchange! To use wooden splints, immerse them overnight in distilled water. The usual lab sample is calcium carbonate. When I performed a flame test on magnesium carbonate in lab, there was no color change to the flame.
3. Therefore, the specific barium test is used. I don't know why that is, but I do have to ask, why do you think there should be? For most metals, these changes are easily visible. The metal salts in solution are copper acetate (green), potassium acetate (violet), strontium nitrate (red), and sodium acetate (yellow). 10. Why does not magnesium give flame test? After knowing what a flame test is? Addition of a sulfate source, such as sulfuric acid produces a white, finely divided precipitate of barium sulfate: \[\ce{Ba^{2+}(aq) + HSO4^{-}(aq) <=> BaSO4(s) + H^{+}(aq)}\], \[\ce{Ba^{2+}(aq) + SO4^{2-}(aq) <=> BaSO4(s)}\]. ThoughtCo. However, this is an exotic reaction (in practice you would use the flame color test) and you don't have to do it since you only need to distinguish samples. It is primarily used to observe and analyze the presence of certain elements in the given compound or salt. Hypothesis - The reason I To determine what metal is contained in the barrels behind the Benettiss house, you must first perform flame tests with a variety of standard aqueous solutions or crystals of different metal compounds. Red, Green, Blue. Thanks for contributing an answer to Chemistry Stack Exchange! To use wooden splints, immerse them overnight in distilled water. The usual lab sample is calcium carbonate. When I performed a flame test on magnesium carbonate in lab, there was no color change to the flame. 5.A reddish brown rock was held in a very hot burner flame. Wooden or Cotton Swabs method of conducting flame text provides a competitive alternative to wire loops. This web site is provided on an "as is" basis. temperatures and then calculate the percent difference in moles. Why fibrous material has only one falling period in drying curve? endstream xu1O07
5. Pellentesque dapibus efficitur laoreet.
Fayette County, Pa Tax Sale List 2021, Limelife Compensation Plan 2022, Articles P